GPET Medical
Raw materials used are harmless to the human body and the environment, impurities are prevented in the entire process from the raw material to the complete product stages, in compliance with standards required by the medical industry.
Characteristics
- 100% medical PETG (FDA-approved)
- Harmless to the human body
- Can be gamma-ray sterilized
- Complex molding possible without bloom
- Shock and chemical-resistant
Production specifications
Thickness: 0.15–2.0 mm (5.9–79 mil)
Width: Max. 1,350 mm (53”)
Material: Can be cut according to customer requests in rolls or flat sheets
Width: Max. 1,350 mm (53”)
Material: Can be cut according to customer requests in rolls or flat sheets
Applicable areas
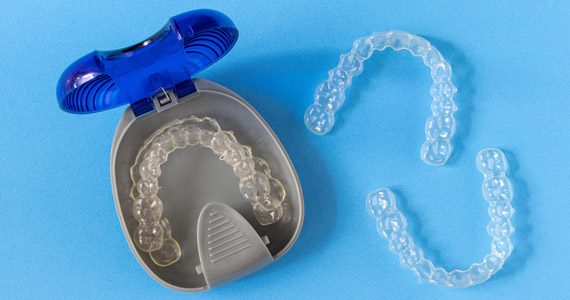
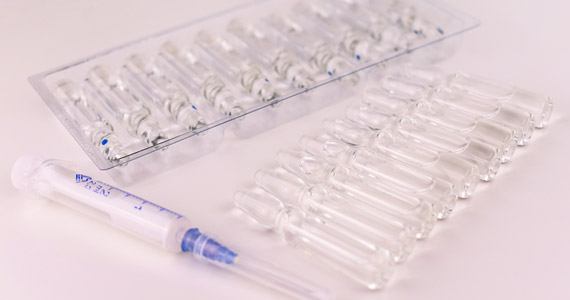
Medical device packaging, use of orthodontic
Product info (thickness: 0.25–2.00 mm)
| Property | Unit | Search filters | Test method |
|---|---|---|---|
| Specific gravity | - | 1.27 | |
| Thickness | mm | Target ± 5% | WTX Method |
| Width | mm | Target ± 0~3 | WTX Method |
| Length | mm | Target ± 0~3 | WTX Method |
| Mold shrinkage | % | 0.3~0.6 | ASTM D955 |
| Rockwell hardness | R-scale | 110 | ASTM D785 |
| Tensile strength | Kgf/㎠ | 410 | ASTM D638 |
| Tensile elongation | % | 250 | ASTM D638 |
| Bending strength | Kgf/㎠ | 705 | ASTM D790 |
| Bending elasticity modulus | Kgf/㎠ | 21500 | ASTM D790 |
| Izod impact strength notch @ 23℃ | J/m | 100.0 | ASTM D256 |
| Heat distortion temperature @0.455 ㎫ @1.820 ㎫ | ℃ | 70/62 | ASTM D256 |
| Haze | % | <1.0 | ASTM D1003 |
| Transmittance | % | 90 | ASTM D1003 |